г. Москва, ул. Привольная, 70, корп. 1

5900 руб.
комплексная чистка зубов,включаяAir FlowКомплексная чистка - ультразвук+Air Flow+полировка+фторирование.Вся процедура состоит из снятия зубных отложений ультразвуком, удаление зубного налёта,а это мягкий и пигментированный плотный зубной налет, зубной камень, «налет курильщиков» при помощи Air-Flow ,полировка и нанесение на зубную эмаль фторсодержащих гелей.
Панорамный снимок
1000 рублей с записью на диск печать на бумагена оборудовании Sirona Одним из основных принципов работы стоматологической клиники «Приват-Денталь» является полноценное обеспечение надежности, гигиеничности и безопасности лечения. Для этого все отделы клиники оснащены высококачественными лечебными материалами и профессиональным оборудованием высшего класса.
Стоматология Люберцы, Жулебино, Москва (ЮВАО)
Очень часто рынок оказания услуг ассоциируется с длительной процедурой, но при этом, как можно наблюдать на практике, - редко ставящей целью достижение фактического результата. И по этой причине наша стоматологическая клиника на Привольной не позиционирует себя как субъект по оказанию услуг. Наши компетентные специалисты не занимаются сервисным обслуживанием, а действительно лечат зубы, демонстрируя высокую степень профессионализма и достигая конкретных целей.
Мы ежедневно выполняем целый комплекс стоматологических процедур в обширном диапазоне, и делаем свою работу без претензий к качеству.
В нашей концепции обслуживания пациентов заложены принципы конструктивного диалога и взаимопонимания. Поэтому мы ни при каких обстоятельствах не навязываем своих предпочтений в определении методов и средств, оставляя своим клиентам право выбора. Следуя нашим принципам, мы никогда не доминируем над мнением наших пациентов, находя в тесном сотрудничестве с ними максимально рациональные решения.
Едва ли не ежедневно в столице начинает функционировать очередная детская стоматология, врачебные стоматологические кабинеты или другие профильные учреждения. Развитие собственной сети наших стоматологических клиник на территории Юго-Восточного АО столицы позволит всем жителям сделать выбор достойного лечащего врача, получить ценные профессиональные консультации и больше узнать о том, как правильно ухаживать за своими зубами. Если Вы ищете достойное обслуживание Люберцах. Жулебино или Москве, то наша стоматология – это Ваш выбор. Наши цены вас приятно удивят.
Протезирование зубов — особенности
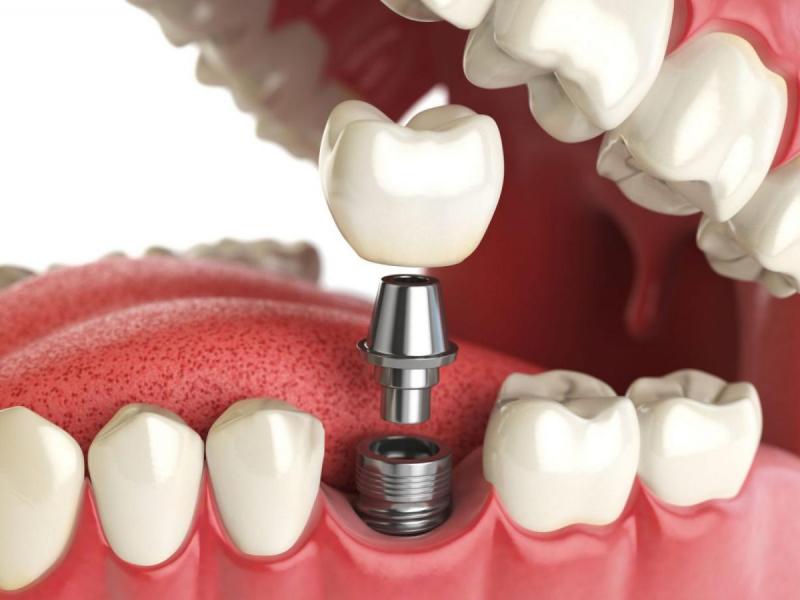
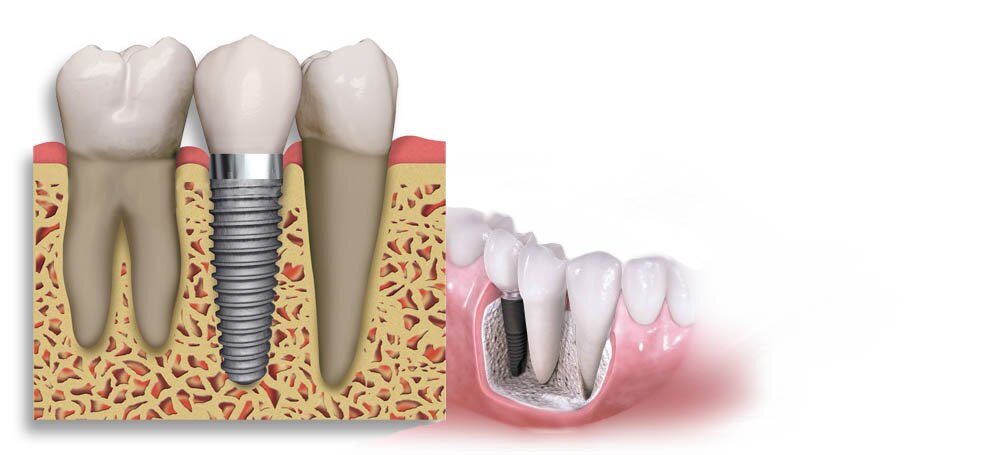
Как известно, в стоматологии есть несколько отделов или более мелких дисциплин/специальностей. Наиболее популярными являются ортодонтия, стоматологическая хирургия и протезирование. На этот раз сосредоточимся на последнем. Что такое протезирование зубов? Что это за метод? Кому подходит услуга протезирования? Каких эффектов можно ожидать после протезирования и в чем его польза?
Что такое протезирование? Определение
Это правда, что мы уже знаем, что протезирование является одной из дисциплин стоматологии, но что это такое? Что такое протезирование и какова цель протезирования?
Что ж, протезирование в основном направлено на реконструкцию зубов и, таким образом, на восстановление правильного прикуса и внешнего вида зубов.
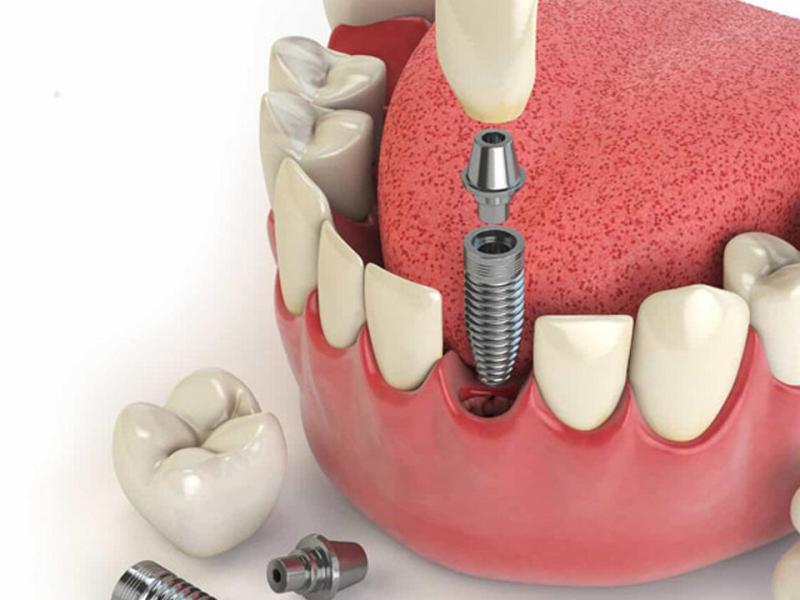
Протезирование — что это и для кого?
Как мы уже установили, протезирование как один из разделов стоматологии занимается реконструкцией зубов.
Поэтому его применяют в ситуации потери или сильного повреждения постоянных зубов, когда стандартные методы консервативной стоматологии не справляются, т.е. не в состоянии справиться с явным отсутствием зубов. Следует отметить, что проблема потери зубов может коснуться как пожилых, так и молодых людей в расцвете сил.
К причинам такого явления можно отнести не только преклонный возраст, но и различные заболевания, травмы или просто пренебрежение гигиеной полости рта.
Поэтому все процедуры протезирования направлены на всех тех, у кого заметное отсутствие зубов, вне зависимости от возраста.
Современные протезы имеют либо подвижную, либо фиксированную форму. Первые когда-то были настолько популярными зубными протезами, что их можно было вынуть изо рта в любой момент. Применяются в случае полной потери зубного ряда или большей части зубов.
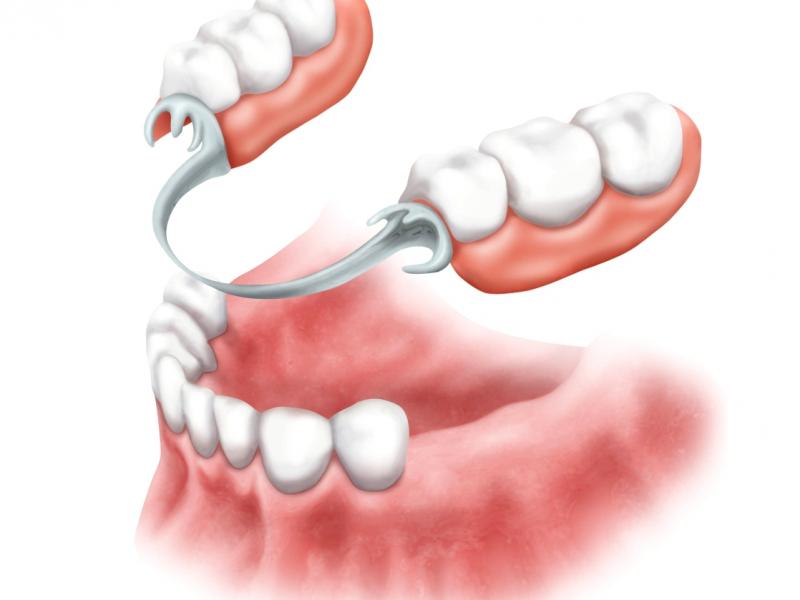
В свою очередь, постоянные реставрации включают мосты, коронки и коронко-корневые вкладки. При этом следует отметить, что каждый случай требует индивидуального подхода и подбора наиболее подходящего метода и ортопедического лечения.
Протезирование — преимущества
Поскольку мы уже разобрались, что такое протезирование, в конце стоит упомянуть о преимуществах прохождения протезирования.
И так, к ним относится восстановление правильной работы прикуса, а то и всей пищеварительной системы, облегчение приема пищи, а также предотвращение потери оставшихся зубов и возникновения полостей в костях. Эстетика не менее важна — протезирование позволяет иметь красивую, сияющую улыбку, несмотря на болезни, травмы, дефекты и возраст.
Добро пожаловать!
Мы рады приветствовать Вас на сайте клиники Приват-Денталь!
Стоматологическая клиника "Приват Денталь" - это новый формат предоставления стоматологических услуг людям, которые ценят свое время, дорожат своим здоровьем, привыкли получать качество в обслуживании и не ведутся на рекламные уловки.
Если всё это о вас - вы попали по адресу. Мы не обещаем качество, мы его обеспечиваем.
ПодробнееО клинике
В данном разделе вы сможете детально познакомиться с нашей клиникой
Сегодня стоматологических клиник в Москве не просто много, а катастрофически много... Чем же мы отличаемся от других? Да очень просто — мы не оказываем стоматологических услуг, но пытаемся качественно лечить зубы.
Мы стараемся помочь нашим пациентам избежать «товарной ловушки», в которую их загоняет рынок услуг.
ПодробнееСтоматология в Жулебино
При любой проблеме с зубами посещение нашей клиники «Приват Денталь» станет первой ступенью на пути к здоровью ротовой полости. Здесь вы получите доступное в бюджетном отношении компетентное сервисное обслуживание в условиях располагающей атмосферы.
Вам не придется ожидать завершения обеденного перерыва или искать врача, работающего в выходные дни – наши специалисты всегда пребывают в готовности оказать помощь всем нашим клиентам. При этом мы окажем любое консультационное и практическое содействие в таких вопросах:
- Профилактика патологий ротовой полости;
- Гигиенический уход за полостью рта в профессиональном ракурсе;
- Методы хирургической стоматологии;
- Терапия стоматологических заболеваний;
- Имплантационные процедуры и протезирование;
- Обслуживание юных пациентов по всем вопросам возрастной стоматологии;
- Ортодонтическое исправление;
- Эстетические операции, включая отбеливание и реставрацию зубов.
Современные продуктивные технологии нашей стоматологии уже доступны во многих населенных пунктах ЮВАО столицы. На карте округа все чаще возникают адреса наших клиник в Реутове, Жулебино, Выхино, Марьино, в Москве, Новокосино и других географических точках.
Во всех этих населенных пунктах жителям стало доступным настоящее профессиональное обслуживание нашими компетентными и опытными стоматологами.
Обратив свое внимание на цены наших стоматологов, Вы поймете, что они трудятся не ради прибыли любым путем, не ради рекламируемой белоснежной улыбки. Их деятельность направлена на то, чтобы Ваши зубы и ротовая полость надолго остались пребывать в здоровом состоянии.
И если Вы нуждаетесь, прежде всего, в здоровье, то наши врачи-стоматологи быстро и профессионально приведут Вас к этой цели. Многочисленные отзывы наших пациентов смогут убедить Вас в дружелюбии, компетентности, опыте и других положительных признаках нашей клиники «Приват Денталь».
А мы будем рады оказать по-настоящему качественные и профессиональные услуги во всех направлениях стоматологии.
- Протезирование зубов: что важно знать
- Имплантация зубов
- Виды анестезии в стоматологии
- Эстетическая стоматология недорого
- Бюгельные протезы: общее понятие и особенности
- Лечение под стоматологическим микроскопом
- Преимущества светоотверждаемых пломб
- Виниры: виды и назначение
Наша стоматология Люберцы на юговостоке (ЮВАО) обслуживает следующие районы и города: Выхино, Жулебино, Котельники, Кожухово, Дзержинский, Реутов, Новокосино, Марьино и многие другие административные округи.

Последние новости
Открытие станции метро Котельники
Уважемые пользователи нашего сайта, рады сообщить Вам о том, что 21 сентября 2015 года состоялось открытие станции метро Котельники. Теперь доехать до нас стало еще проще и удобнее!
ПодробнееС днем стоматолога!
Международный день стоматолога отмечается 9 февраля во всем мире. Мастеров сверла боятся все - от детей до взрослых. Противный звук от сверления зуба, острая боль при удалении нерва без наркоза и кровавые плевки - вот самые запоминающие картинки, которые рисуют в своем сознании потенциальные пациенты стоматологического кабинета...
ПодробнееОткрыт сайт клиники Приват Денталь!
Мы доступны для Вас и в интернет-пространстве! Рады сообщить, что сайт клиники "Приват Денталь" в Жулебино открыт и рад приветствовать своих посетителей!
ПодробнееРелаксационный раздел сайта
Плох тот специалист, который не может как следует посмеяться над собой и своей профессией.. Чувство юмора - незаменимая часть ежедневного существования и в жизни, и в работе, мы ценим это качество и рады обнаруживать его у себя...
ПодробнееС Новым годом и Рождеством!
Дорогие наши клиенты и посетители сайта клиники "Приват Денталь"! Поздравляем вас с наступающими новогодними праздниками и желаем вам и вашим близким искренних радостей и белоснежных улыбок, пушистого снега и чистого неба, домашнего уюта и семейного тепла! До встречи в новом году!
ПодробнееТеперь мы работаем для вас и в выходные!
Дорогие наши клиенты! Рады сообщить вам, что теперь наш режим работы стал более лояльным, а значит, более удобным! Мы принимаем вас и в выходные дни, а значит возможности заняться собой и своими зубами стало больше!
ПодробнееВ нашей копилке сертификатов о прохождении спецкурсов прибыло!
Рады сообщить, что в нашей копилке сертификатов о прохождении спецкурсов нашими сотрудниками прибыло! В минувшие выходные Главный врач Клиники "Приват Денталь" Жданов Валерий Владимирович прослушал практический семинар "ОРТОПЕДИЯ на имплантатах
Подробнее

